How To Draw Up Lorazepam Injection
Editor-In-Primary: C. Michael Gibson, 1000.Due south., M.D. [i]; Acquaintance Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional wellness care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, non a tool for any class of healthcare commitment. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does non promote the administration of any medication or device that is non consequent with its labeling. Delight read our full disclaimer here.
Overview
Lorazepam (injection) is a general coldhearted that is FDA approved for the treatment of anxiety, indisposition, due to anxiety or situational stress, premedication for anesthetic procedure, condition epilepticus. Mutual adverse reactions include neurologic: asthenia (iv.2% ), dizziness (vi.9% ), sedated (15.nine% ), unsteadiness present (iii.iv%), psychiatric: depression.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Alcohol withdrawal syndrome: 2 mg orally every 6 hr for 4 doses, and then 1 mg every six 60 minutes for 8 doses.
- Feet: initial, two to 3 mg/day orally divided into 2 to 3 daily doses.
- Anxiety: maintenance, 2 to 6 mg/twenty-four hours orally divided into 2 to 3 daily doses; dose may vary from 1 to 10 mg/day.
- Chemotherapy-induced nausea and vomiting; prophylaxis: a single dose of 0.025 to 0.05 mg/kg (max 4 mg) IM OR 4 given slowly (ii mg/min) thirty to 35 min prior to receiving chemotherapy; this dose may be supplemented with oral lorazepam 1 to 2 mg/60 minutes as needed.
- Insomnia, due to anxiety or situational stress: 2 to four mg orally at bedtime.
- Premedication for anesthetic procedure: 0.05 mg/kg IM (max 4 mg) 2 hours before procedure.
- Premedication for anesthetic procedure: 0.044 mg/kg IV OR two mg (whichever is less); MAX dose 0.05 mg/kg Four OR 4 mg (whichever is less) xv to 20 minutes before the surgical process.
- Sedation: (intermittent) 0.02 to 0.06 mg/kg Iv every 2 to half-dozen hours.
- Sedation: (continuous infusion) 0.01 to 0.ane mg/kg/hr IV.
- Status epilepticus: four mg Iv given slowly at 2 mg/min, may echo dose in 10 to fifteen min if needed; IM dosing may be used, only 4 dosing is preferred.
Off-Label Utilise and Dosage (Adult)
Guideline-Supported Use
- Anxiety
- Insomnia, due to feet or situational stress
- Premedication for anesthetic procedure.
- Condition epilepticus.
Non–Guideline-Supported Employ
- Agitation - psychotic disorder
- Booze withdrawal syndrome
- Chemotherapy-induced nausea and vomiting; prophylaxis
- Sedation
- Seizure
- Seizure, drug-induced; prophylaxis
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
In that location is limited information regarding Lorazepam (injection) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Utilize
- There is express information almost Off-Characterization Guideline-Supported Apply of Lorazepam in pediatric patients.
Non–Guideline-Supported Use
- There is express data nearly Off-Label Non–Guideline-Supported Use of Lorazepam in pediatric patients.
Contraindications
- Ativan Injection is contraindicated in patients with a known sensitivity to benzodiazepines or its vehicle (polyethylene glycol, propylene glycol, and benzyl alcohol), in patients with acute narrow-bending glaucoma, or in patients with sleep apnea syndrome. It is also contraindicated in patients with severe respiratory insufficiency, except in those patients requiring relief of anxiety and/or diminished think of events while beingness mechanically ventilated. The use of Ativan Injection intra-arterially is contraindicated because, equally with other injectable benzodiazepines, inadvertent intra-arterial injection may produce arteriospasm resulting in gangrene which may require amputation (see Warnings)
Warnings
Use in Status Epilepticus
Management of Status Epilepticus
- Condition epilepticus is a potentially life-threatening condition associated with a high risk of permanent neurological damage, if inadequately treated. The treatment of status, notwithstanding, requires far more than the assistants of an anticonvulsant amanuensis. Information technology involves ascertainment and management of all parameters critical to maintaining vital role and the capacity to provide back up of those functions as required. Ventilatory support must exist readily available. The apply of benzodiazepines, like Ativan Injection, is commonly only one step of a complex and sustained intervention which may crave additional interventions (eastward.g., concomitant intravenous administration of phenytoin). Because condition epilepticus may effect from a correctable acute cause such equally hypoglycemia, hyponatremia, or other metabolic or toxic derangement, such an aberration must exist immediately sought and corrected. Furthermore, patients who are susceptible to further seizure episodes should receive adequate maintenance antiepileptic therapy.
- Any health care professional who intends to treat a patient with condition epilepticus should be familiar with this package insert and the pertinent medical literature concerning electric current concepts for the treatment of status epilepticus. A comprehensive review of the considerations critical to the informed and prudent direction of status epilepticus cannot exist provided in drug product labeling. The archival medical literature contains many informative references on the management of status epilepticus, amid them the written report of the working group on status epilepticus of the Epilepsy Foundation of America "Treatment of Convulsive Condition Epilepticus" (JAMA 1993; 270:854-859). Equally noted in the report just cited, it may be useful to consult with a neurologist if a patient fails to reply (e.1000., fails to regain consciousness).
- For the treatment of status epilepticus, the usual recommended dose of Ativan Injection is 4 mg given slowly (two mg/min) for patients 18 years and older. If seizures cease, no additional Ativan Injection is required. If seizures continue or recur later a 10- to 15- minute ascertainment period, an boosted 4 mg intravenous dose may be slowly administered. Experience with further doses of Ativan is very express. The usual precautions in treating status epilepticus should be employed. An intravenous infusion should be started, vital signs should exist monitored, an unobstructed airway should be maintained, and artificial ventilation equipment should be bachelor.
Respiratory Depression
- The most of import chance associated with the use of Ativan Injection in status epilepticus is respiratory depression. Appropriately, airway patency must exist assured and respiration monitored closely. Ventilatory back up should be given as required.
Excessive Sedation
- Because of its prolonged elapsing of action, the prescriber should exist warning to the possibility, peculiarly when multiple doses have been given, that the allaying effects of lorazepam may add together to the damage of consciousness seen in the post-ictal state.
Preanesthetic Apply
- Airway obstruction may occur in heavily sedated patients. intravenous lorazepam at any dose, when given either alone or in combination with other drugs administered during anesthesia, may produce heavy sedation; therefore, equipment necessary to maintain a patent airway and to support respiration/ventilation should be bachelor.
- Equally is true of like CNS-acting drugs, the decision as to when patients who have received injectable lorazepam, particularly on an outpatient basis, may once more operate machinery, bulldoze a motor vehicle, or appoint in hazardous or other activities requiring attention and coordination must be individualized. It is recommended that no patient appoint in such activities for a period of 24 to 48 hours or until the effects of the drug, such every bit drowsiness, have subsided, whichever is longer. Impairment of performance may persist for greater intervals because of extremes of historic period, concomitant use of other drugs, stress of surgery, or the full general condition of the patient.
- Clinical trials have shown that patients over the age of 50 years may take a more profound and prolonged sedation with intravenous lorazepam (see also Dosage and administration, Preanesthetic).
- As with all key-nervous-system-depressant drugs, care should be exercised in patients given injectable lorazepam equally premature ambulation may result in injury from falling.
- There is no added beneficial effect from the improver of scopolamine to injectable lorazepam, and their combined issue may result in an increased incidence of sedation, hallucination and irrational behavior.
General (All Uses)
- Prior to intravenous use, ativan injection must be diluted with an equal amount of compatible diluent (see dosage and administration). intravenous injection should be fabricated slowly and with repeated aspiration. care should be taken to determine that any injection will not exist intra-arterial and that perivascular extravasation will non take identify. in the event that a patient complains of pain during intended intravenous injection of ativan injection, the injection should be stopped immediately to decide if intra-arterial injection or perivascular extravasation has taken place.
- Since the liver is the nearly probable site of conjugation of lorazepam and since excretion of conjugated lorazepam (glucuronide) is a renal part, this drug is not recommended for use in patients with hepatic and/or renal failure. Ativan should exist used with caution in patients with balmy-to-moderate hepatic or renal affliction (run across Dosage and administration).
Pregnancy
- Ativan may cause fetal impairment when administered to pregnant women. Ordinarily, Ativan injection should non be used during pregnancy except in serious or life-threatening conditions where safer drugs cannot be used or are ineffective. status epilepticus may stand for such a serious and life-threatening condition.
- An increased risk of built malformations associated with the use of minor tranquilizers (chlordiazepoxide, diazepam and meprobamate) during the outset trimester of pregnancy has been suggested in several studies. In humans, blood levels obtained from umbilical cord blood indicate placental transfer of lorazepam and lorazepam glucuronide.
- Reproductive studies in animals were performed in mice, rats, and two strains of rabbits. Occasional anomalies (reduction of tarsals, tibia, metatarsals, malrotated limbs, gastroschisis, malformed skull, and microphthalmia) were seen in drug-treated rabbits without relationship to dosage. Although all of these anomalies were not present in the concurrent control group, they have been reported to occur randomly in historical controls. At doses of forty mg/kg orally or 4 mg/kg intravenously and higher, there was bear witness of fetal resorption and increased fetal loss in rabbits which was not seen at lower doses.
- The possibility that a woman of childbearing potential may be meaning at the time of therapy should exist considered.
- In that location are insufficient information regarding obstetrical safety of parenteral lorazepam, including apply in cesarean section. Such use, therefore, is not recommended.
Endoscopic Procedures
- In that location are insufficient data to support the use of Ativan Injection for outpatient endoscopic procedures. Inpatient endoscopic procedures require acceptable recovery room observation time.
- When Ativan Injection is used for peroral endoscopic procedures; adequate topical or regional anesthesia is recommended to minimize reflex activity associated with such procedures.
Agin Reactions
Clinical Trials Experience
Status Epilepticus
- The most of import adverse clinical result acquired by the employ of Ativan Injection is respiratory low (encounter Warnings).
- The agin clinical events most commonly observed with the apply of Ativan Injection in clinical trials evaluating its utilise in condition epilepticus were hypotension, somnolence, and respiratory failure.
Incidence in Controlled Clinical Trials
- All adverse events were recorded during the trials by the clinical investigators using terminology of their own choosing. Similar types of events were grouped into standardized categories using modified COSTART lexicon terminology. These categories are used in the table and listings beneath with the frequencies representing the proportion of individuals exposed to Ativan Injection or to comparative therapy.
- The prescriber should be aware that these figures cannot be used to predict the frequency of adverse events in the course of usual medical practise where patient characteristics and other factors may differ from those prevailing during clinical studies. Similarly, the cited frequencies cannot be straight compared with figures obtained from other clinical investigators involving dissimilar treatment, uses, or investigators. An inspection of these frequencies, nevertheless, does provide the prescribing physician with one basis to estimate the relative contribution of drug and nondrug factors to the adverse result incidences in the population studied.
- Commonly Observed Adverse Events in a Controlled Dose-Comparison Clinical Trial
- Table i lists the treatment-emergent adverse events that occurred in the patients treated with Ativan Injection in a dose-comparison trial of Ativan 1 mg, 2 mg, and 4 mg.

This image is provided by the National Library of Medicine.
Ordinarily Observed Adverse Events in Agile-Controlled Clinical Trials
- In two studies, patients who completed the form of treatment for status epilepticus were permitted to be reenrolled and to receive treatment for a second status episode, given that there was a sufficient interval between the 2 episodes. Rubber was determined from all handling episodes for all intent-to-treat patients, i.due east., from all "patient-episodes." Table ii lists the treatment-emergent adverse events that occurred in at least 1% of the patient-episodes in which Ativan Injection or diazepam was given. The table represents the pooling of results from the ii controlled trials.
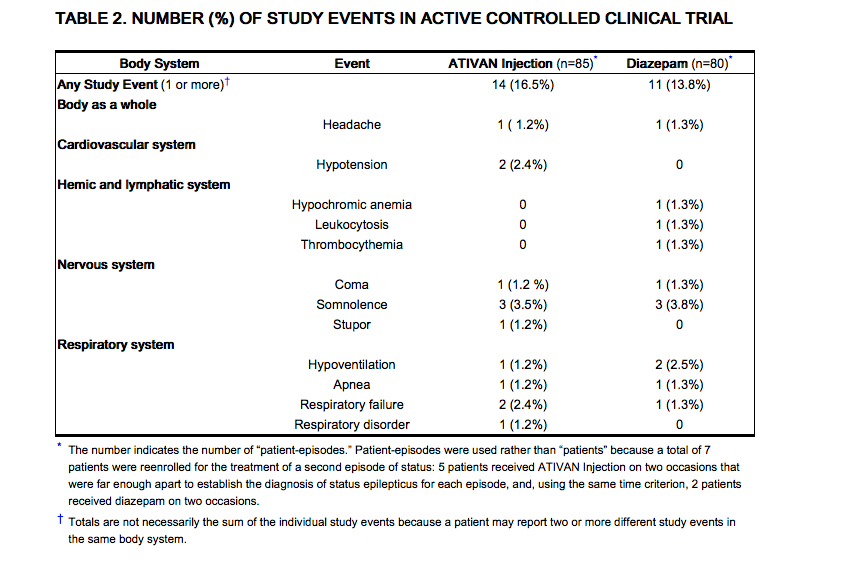
This image is provided by the National Library of Medicine.
- These trials were non designed or intended to demonstrate the comparative condom of the two treatments.
- The overall adverse experience profile for Ativan was like between women and men. There are insufficient information to support a statement regarding the distribution of adverse events past race. Generally, age greater than 65 years may be associated with a greater incidence of central-nervous-system depression and more respiratory low.
Other Events Observed During the Pre-Marketing Evaluation of Ativan Injection for the Handling of Status Epilepticus
- Ativan Injection, active comparators, and Ativan Injection in combination with a comparator were administered to 488 individuals during controlled and open-label clinical trials. Because of reenrollments, these 488 patients participated in a total of 521 patient-episodes. Ativan Injection alone was given in 69% of these patient-episodes (northward=360). The safety information below is based on information bachelor from 326 of these patient-episodes in which Ativan Injection was given lone.
- All adverse events that were seen in one case are listed, except those already included in previous listings (Table 1 and Table 2).
- Study events were classified by torso system in descending frequency by using the following definitions: frequent adverse events were those that occurred in at to the lowest degree 1/100 individuals; infrequent study events were those that occurred in one/100 to ane/1000 individuals.
Frequent and Infrequent Written report Events

This epitome is provided by the National Library of Medicine.
Preanesthetic
Central Nervous System
- The nearly frequent adverse drug consequence reported with injectable lorazepam is cardinal-nervous-organisation depression. The incidence varied from i study to another, depending on the dosage, road of administration, use of other key-nervous-system depressants, and the investigator's opinion concerning the degree and duration of desired sedation. Excessive sleepiness and drowsiness were the almost common consequences of CNS depression. This interfered with patient cooperation in approximately six% (25/446) of patients undergoing regional anesthesia, causing difficulty in assessing levels of anesthesia. Patients over 50 years of age had a higher incidence of excessive sleepiness or drowsiness when compared with those nether 50 (21/106 versus 24/245) when lorazepam was given intravenously (see DOSAGE AND Administration). On rare occasion (3/1580) the patient was unable to give personal identification in the operating room on inflow, and one patient fell when attempting premature ambulation in the postoperative period.
- Symptoms such every bit restlessness, defoliation, depression, crying, sobbing, and delirium occurred in about 1.3% (20/1580). One patient injured himself by picking at his incision during the immediate postoperative period.
- Hallucinations were present in nearly 1% (14/1580) of patients and were visual and self-limiting.
- An occasional patient complained of dizziness, diplopia and/or blurred vision. Depressed hearing was infrequently reported during the pinnacle-effect period.
- An occasional patient had a prolonged recovery room stay, either because of excessive sleepiness or because of some course of inappropriate behavior. The latter was seen nigh commonly when scopolamine was given concomitantly every bit a premedicant. Express data derived from patients who were discharged the day after receiving injectable lorazepam showed ane patient complained of some unsteadiness of gait and a reduced ability to perform complex mental functions. Enhanced sensitivity to alcoholic beverages has been reported more than than 24 hours after receiving injectable lorazepam, similar to experience with other benzodiazepines.
Local Effects
- Intramuscular injection of lorazepam has resulted in pain at the injection site, a sensation of called-for, or observed redness in the same area in a very variable incidence from one study to another. The overall incidence of pain and burning in patients was about 17% (146/859) in the firsthand postinjection menstruum and about 1.4% (12/859) at the 24-hour ascertainment time. Reactions at the injection site (redness) occurred in approximately 2% (17/859) in the immediate postinjection period and were nowadays 24 hours later on in about 0.eight% (seven/859).
- Intravenous administration of lorazepam resulted in painful responses in 13/771 patients or approximately 1.6% in the immediate postinjection period, and 24 hours later on 4/771 patients or nigh 0.5% nonetheless complained of hurting. Redness did not occur immediately following intravenous injection but was noted in 19/771 patients at the 24-hour ascertainment period. This incidence is similar to that observed with an intravenous infusion before lorazepam is given. Intra-arterial injection may produce arteriospasm resulting in gangrene which may require amputation (see Contraindications).
Cardiovascular Organization
- Hypertension (0.one%) and hypotension (0.1%) have occasionally been observed after patients have received injectable lorazepam.
Respiratory System
- Five patients (5/446) who underwent regional anesthesia were observed to accept airway obstruction. This was believed due to excessive sleepiness at the time of the process and resulted in temporary hypoventilation. In this instance, appropriate airway management may become necessary (see also Clinical pharmacology, warnings and precautions).
Other Agin Experiences
- Peel rash, nausea and vomiting have occasionally been noted in patients who have received injectable lorazepam combined with other drugs during anesthesia and surgery.
Paradoxical Reactions
- As with all benzodiazepines, paradoxical reactions such as stimulation, mania, irritability, restlessness, agitation, assailment, psychosis, hostility, rage, or hallucinations may occur in rare instances and in an unpredictable fashion. In these instances, further utilise of the drug in these patients should exist considered with caution (see Precautions, General).
Postmarketing Experience
Postmarketing Reports
- Voluntary reports of other adverse events temporally associated with the apply of Ativan (lorazepam) Injection that have been received since market place introduction and that may have no causal relationship with the use of Ativan Injection include the following: astute brain syndrome, bedevilment of pheochromocytoma, amnesia, apnea/respiratory abort, arrhythmia, bradycardia, brain edema, coagulation disorder, coma, earthquake, gastrointestinal hemorrhage, heart abort/failure, center block, liver damage, lung edema, lung hemorrhage, nervousness, neuroleptic cancerous syndrome, paralysis, pericardial effusion, pneumothorax, pulmonary hypertension, tachycardia, thrombocytopenia, urinary incontinence, ventricular arrhythmia.
- Fatalities also take been reported, normally in patients on concomitant medications (east.yard., respiratory depressants) and/or with other medical atmospheric condition (due east.grand., obstructive sleep apnea).
Drug Interactions
In that location is limited information regarding Lorazepam (injection) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): D
- Ativan may cause fetal damage when administered to pregnant women. Ordinarily, Ativan Injection should not exist used during pregnancy except in serious or life-threatening conditions where safer drugs cannot exist used or are ineffective. Status epilepticus may represent such a serious and life-threatening status.
- An increased risk of congenital malformations associated with the use of minor tranquilizers (chlordiazepoxide, diazepam and meprobamate) during the offset trimester of pregnancy has been suggested in several studies. In humans, blood levels obtained from umbilical cord blood signal placental transfer of lorazepam and lorazepam glucuronide.
- Reproductive studies in animals were performed in mice, rats, and two strains of rabbits. Occasional anomalies (reduction of tarsals, tibia, metatarsals, malrotated limbs, gastroschisis, malformed skull, and microphthalmia) were seen in drug-treated rabbits without human relationship to dosage. Although all of these anomalies were not nowadays in the concurrent control group, they accept been reported to occur randomly in historical controls. At doses of 40 mg/kg orally or 4 mg/kg intravenously and college, there was prove of fetal resorption and increased fetal loss in rabbits which was not seen at lower doses.
- The possibility that a woman of childbearing potential may be pregnant at the time of therapy should exist considered.
- There are insufficient data regarding obstetrical prophylactic of parenteral lorazepam, including apply in cesarean section. Such use, therefore, is non recommended.
Pregnancy Category (AUS): There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Lorazepam (injection) in women who are pregnant.
Labor and Delivery
- In that location are insufficient data to support the use of Ativan (lorazepam) Injection during labor and delivery, including cesarean section; therefore, its use in this clinical circumstance is not recommended.
Nursing Mothers
- Lorazepam has been detected in human chest milk. Therefore, lorazepam should not be administered to nursing mothers because, like other benzodiazepines, the possibility exists that lorazepam may sedate or otherwise adversely affect the babe.
Pediatric Employ
Neonates (Nascency to 1 month)
- Following a unmarried 0.05 mg/kg (due north=four) or 0.i mg/kg (n=6) intravenous dose of lorazepam, mean total clearance normalized to body weight was reduced by eighty% compared to normal adults, terminal half-life was prolonged three-fold, and volume of distribution was decreased by 40% in neonates with asphyxia neonatorum compared to normal adults. All neonates were of ≥37 weeks of gestational age.
Infants (1 Calendar month UP TO ii Years)
- There is no information on the pharmacokinetic profile of lorazepam in infants in the age range of one calendar month to 2 years.
Children (2 Years TO 12 Years)
- Total (bound and unbound) lorazepam had a l% higher mean volume of distribution (normalized to torso-weight) and a thirty% longer mean one-half-life in children with acute lymphocytic leukemia in complete remission (2 to 12 years, north=37) compared to normal adults (n=10). Unbound lorazepam clearance normalized to body-weight was comparable in children and adults.
Geriatic Apply
- Following single intravenous doses of ane.5 to 3 mg of Ativan Injection, mean total trunk clearance of lorazepam decreased by 20% in xv elderly subjects of 60 to 84 years of age compared to that in 15 younger subjects of 19 to 38 years of historic period. Consequently, no dosage aligning appears to be necessary in elderly subjects based solely on their age
Gender
- Gender has no effect on the pharmacokinetics of lorazepam.
Race
- Young Americans (n=15) and Japanese subjects (due north=7) had very comparable mean total clearance value of ane.0 mL/min/kg. However, elderly Japanese subjects had a 20% lower hateful total clearance than elderly Americans, 0.59 mL/min/kg vs 0.77 mL/min/kg, respectively.
Renal Impairment
- Because the kidney is the primary route of elimination of lorazepam glucuronide, renal impairment would be expected to compromise its clearance. This should have no direct effect on the glucuronidation (and inactivation) of lorazepam. At that place is a possibility that the enterohepatic circulation of lorazepam glucuronide leads to a reduced efficiency of the internet clearance of lorazepam in this population.
- Six normal subjects, six patients with renal damage (Clcr of 22±9 mL/min), and four patients on chronic maintenance hemodialysis were given single 1.5 to iii.0 mg intravenous doses of lorazepam. Mean volume of distribution and final half-life values of lorazepam were 40% and 25% college, respectively, in renally impaired patients than in normal subjects. Both parameters were 75% higher in patients undergoing hemodialysis than in normal subjects. Overall, though, in this group of subjects the mean total clearance of lorazepam did not alter. Nearly viii% of the administered intravenous dose was removed as intact lorazepam during the six-60 minutes dialysis session.
- The kinetics of lorazepam glucuronide were markedly afflicted by renal dysfunction. The hateful terminal half-life was prolonged by 55% and 125% in renally impaired patients and patients under hemodialysis, respectively, as compared to normal subjects. The mean metabolic clearance decreased past 75% and 90% in renally dumb patients and patients under hemodialysis, respectively, as compared with normal subjects. Almost 40% of the administered lorazepam intravenous dose was removed as glucuronide conjugate during the 6-hr dialysis session.
Hepatic Impairment
- Because cytochrome oxidation is non involved with the metabolism of lorazepam, liver disease would not be expected to have an effect on metabolic clearance. This prediction is supported by the observation that post-obit a single 2 mg intravenous dose of lorazepam, cirrhotic male person patients (n=13) and normal male subjects (n=xi) exhibited no substantive divergence in their ability to clear lorazepam.
Females of Reproductive Potential and Males
There is no FDA guidance on the apply of Lorazepam (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance i the use of Lorazepam (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is express information regarding Lorazepam (injection) Assistants in the drug characterization.
Monitoring
There is limited information regarding Lorazepam (injection) Monitoring in the drug label.
4 Compatibility
There is limited data regarding the compatibility of Lorazepam (injection) and IV administrations.
Overdosage
Symptoms
- Overdosage of benzodiazepines is usually manifested by varying degrees of central-nervous-system depression, ranging from drowsiness to coma. In mild cases symptoms include drowsiness, mental confusion and lethargy. In more serious examples, symptoms may include ataxia, hypotonia, hypotension, hypnosis, stages i (one) to 3 (3) coma, and, very rarely, death.
Treatment
- Treatment of overdosage is mainly supportive until the drug is eliminated from the body. Vital signs and fluid residual should be carefully monitored in conjunction with close observation of the patient. An adequate airway should exist maintained and assisted respiration used as needed. With normally functioning kidneys, forced diuresis with intravenous fluids and electrolytes may accelerate emptying of benzodiazepines from the torso. In add-on, osmotic diuretics, such equally mannitol, may be effective as adjunctive measures. In more critical situations, renal dialysis and exchange blood transfusions may be indicated. Lorazepam does not appear to be removed in significant quantities by dialysis, although lorazepam glucuronide may be highly dialyzable. The value of dialysis has not been fairly determined for lorazepam.
- The benzodiazepine antagonist flumazenil may be used in hospitalized patients every bit an adjunct to, not as a substitute for, proper management of benzodiazepine overdose. The prescriber should exist aware of a hazard of seizure in clan with flumazenil treatment, especially in long-term benzodiazepine users and in cyclic antidepressant overdose. The complete flumazenil package insert including Contraindications, Warnings and Precautions should be consulted prior to apply.
Pharmacology
 | |
 | |
| Lorazepam (injection) | |
| Systematic (IUPAC) proper name | |
| (RS)-vii-Chloro-5-(2-chlorophenyl)-three-hydroxy-one,3-dihydro-2H-1,iv-benzodiazepin-2-one | |
| Identifiers | |
| CAS number | |
| ATC code | N05 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 321.ii g/mol |
| SMILES | & |
| Synonyms | O-Chloroxazepam, Fifty-Lorazepam Acetate |
| Pharmacokinetic data | |
| Bioavailability | 85% of oral dose |
| Metabolism | Hepatic glucuronidation |
| Half life | ix–16 hours[i] [2] [3] |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. | C(AU) D(US) |
| Legal status | Prescription Only (S4)(AU) Schedule IV(CA) ?(UK) Schedule Iv(US) |
| Dependence Liability | Moderate |
| Routes | Oral, intramuscular, intravenous, sublingual, and transdermal |
Machinery of Activeness
- Lorazepam interacts with the γ-aminobutyric acid (GABA)-benzodiazepine receptor complex, which is widespread in the encephalon of humans too as other species. This interaction is presumed to be responsible for lorazepam's mechanism of action. Lorazepam exhibits relatively high and specific affinity for its recognition site but does non readapt GABA. Zipper to the specific bounden site enhances the affinity of GABA for its receptor site on the same receptor complex. The pharmacodynamic consequences of benzodiazepine agonist actions include antianxiety effects, sedation, and reduction of seizure activeness. The intensity of action is directly related to the caste of benzodiazepine receptor occupancy.
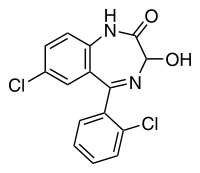
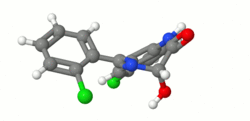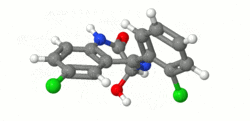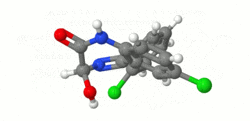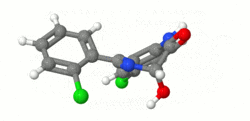
Structure
- Lorazepam, a benzodiazepine with antianxiety, sedative, and anticonvulsant effects, is intended for the intramuscular or intravenous routes of administration. It has the chemical formula: 7-chloro-5(2-chlorophenyl)-1,iii-dihydro-3-hydroxy-2H-1, 4-benzodiazepin-2-1. The molecular weight is 321.16, and the C.A.S. No. is [846-49-i]. The structural formula is:

This image is provided by the National Library of Medicine.
- Lorazepam is a most white pulverization almost insoluble in water. Each mL of sterile injection contains either 2.0 or 4.0 mg of lorazepam, 0.18 mL polyethylene glycol 400 in propylene glycol with two.0% benzyl alcohol as preservative.
Pharmacodynamics
Effects in Pre-Operative Patients
- Intravenous or intramuscular administration of the recommended dose of two mg to 4 mg of Ativan Injection to adult patients is followed by dose-related effects of sedation (sleepiness or drowsiness), relief of preoperative anxiety, and lack of recall of events related to the day of surgery in the majority of patients. The clinical sedation (sleepiness or drowsiness) thus noted is such that the majority of patients are able to respond to elementary instructions whether they give the appearance of existence awake or asleep. The lack of recall is relative rather than absolute, every bit determined under atmospheric condition of conscientious patient questioning and testing, using props designed to enhance retrieve. The majority of patients under these reinforced weather condition had difficulty recalling perioperative events or recognizing props from before surgery. The lack of remember and recognition was optimum within ii hours following intramuscular assistants and 15 to 20 minutes after intravenous injection.
- The intended furnishings of the recommended developed dose of Ativan Injection usually last 6 to viii hours. In rare instances, and where patients received greater than the recommended dose, excessive sleepiness and prolonged lack of recall were noted. Equally with other benzodiazepines, unsteadiness, enhanced sensitivity to CNS-depressant effects of ethyl alcohol and other drugs were noted in isolated and rare cases for greater than 24 hours.
Physiologic Effects in Healthy Adults
- Studies in salubrious adult volunteers reveal that intravenous lorazepam in doses up to 3.5 mg/70 kg does non alter sensitivity to the respiratory stimulating effect of carbon dioxide and does not enhance the respiratory-depressant effects of doses of meperidine up to 100 mg/seventy kg (as well determined by carbon dioxide challenge) as long as patients remain sufficiently awake to undergo testing. Upper airway obstruction has been observed in rare instances where the patient received greater than the recommended dose and was excessively sleepy and difficult to arouse (run across Warnings and adverse reactions).
- Clinically employed doses of Ativan Injection do not greatly affect the circulatory system in the supine position or employing a 70-degree tilt test. Doses of eight mg to x mg of intravenous lorazepam (2 to 2-1/two times the maximum recommended dosage) will produce loss of lid reflexes inside 15 minutes.
- Studies in 6 healthy young adults who received lorazepam injection and no other drugs revealed that visual tracking (the ability to go along a moving line centered) was impaired for a mean of 8 hours following administration of four mg of intramuscular lorazepam and 4 hours post-obit assistants of 2 mg intramuscularly with considerable subject area variation. Like findings were noted with pentobarbital, 150 and 75 mg. Although this study showed that both lorazepam and pentobarbital interfered with heart-paw coordination, the data are insufficient to predict when it would be safe to operate a motor vehicle or appoint in a chancy occupation or sport.
Pharmacokinetics
Absorption
Intravenous
- A 4-mg dose provides an initial concentration of approximately seventy ng/mL.
Intramuscular
- Following intramuscular administration, lorazepam is completely and rapidly absorbed reaching peak concentrations within iii hours. A 4-mg dose provides a Cmax of approximately 48 ng/mL. Following administration of one.5 to 5.0 mg of lorazepam IM, the amount of lorazepam delivered to the circulation is proportional to the dose administered.
Distribution/Metabolism/Elimination
- At clinically relevant concentrations, lorazepam is 91±two% bound to plasma proteins; its volume of distribution is approximately 1.3 L/kg. Unbound lorazepam penetrates the blood/brain barrier freely by passive diffusion, a fact confirmed by CSF sampling. Following parenteral administration, the final half-life and total clearance averaged 14±five hours and ane.1±0.4 mL/min/kg, respectively.
- Lorazepam is extensively conjugated to the iii-O-phenolic glucuronide in the liver and is known to undergo enterohepatic recirculation. Lorazepam glucuronide is an inactive metabolite and is eliminated mainly past the kidneys.
- Following a single 2-mg oral dose of 14C-lorazepam to viii salubrious subjects, 88±4% of the administered dose was recovered in urine and 7±2% was recovered in feces. The percent of administered dose recovered in urine equally lorazepam glucuronide was 74±four%. Only 0.iii% of the dose was recovered as unchanged lorazepam, and the balance of the radioactive decay represented pocket-sized metabolites.
Nonclinical Toxicology
There is limited information regarding Lorazepam (injection) Nonclinical Toxicology in the drug characterization.
Clinical Studies
- The effectiveness of Ativan Injection in status epilepticus was established in two multi-eye controlled trials in 177 patients. With rare exceptions, patients were between 18 and 65 years of age. More than one-half the patients in each report had tonic-clonic condition epilepticus; patients with simple fractional and complex partial condition epilepticus comprised the rest of the population studied, along with a smaller number of patients who had absence status.
- One written report (n=58) was a double-bullheaded active-command trial comparing Ativan Injection and diazepam. Patients were randomized to receive Ativan 2 mg IV (with an boosted ii mg 4 if needed) or diazepam v mg IV (with an boosted 5 mg IV if needed). The principal outcome measure was a comparison of the proportion of responders in each treatment group, where a responder was defined as a patient whose seizures stopped within 10 minutes after treatment and who continued seizure-free for at least an additional 30 minutes. 20-four of the 30 (lxxx%) patients were deemed responders to Ativan and 16/28 (57%) patients were deemed responders to diazepam (p=0.04). Of the 24 Ativan responders, 23 received both 2 mg infusions.
- Not-responders to Ativan iv mg were given an additional 2 to 4 mg Ativan; non-responders to diazepam x mg were given an additional 5 to 10 mg diazepam. Subsequently this additional dose administration, 28/30 (93%) of patients randomized to Ativan and 24/28 (86%) of patients randomized to diazepam were deemed responders, a deviation that was non statistically significant.
- Although this report provides support for the efficacy of Ativan as the treatment for status epilepticus, it cannot speak reliably or meaningfully to the comparative performance of either diazepam (Valium) or lorazepam (Ativan Injection) under the conditions of bodily utilise.
- A second written report (northward=119) was a double-blind dose-comparison trial with 3 doses of Ativan Injection: 1 mg, 2 mg, and 4 mg. Patients were randomized to receive one of the 3 doses of Ativan. The chief upshot and definition of responder were as in the showtime study. Twenty-five of 41 patients (61%) responded to one mg Ativan; 21/37 patients (57%) responded to 2 mg Ativan; and 31/41 (76%) responded to 4 mg Ativan. The p-value for a statistical test of the divergence between the Ativan 4 mg dose group and the Ativan 1-mg dose grouping was 0.08 (ii-sided). Information from all randomized patients were used in this exam.
- Although analyses failed to detect an effect of age, sex, or race on the effectiveness of Ativan in status epilepticus, the numbers of patients evaluated were besides few to let a definitive conclusion nigh the role these factors may play.
How Supplied
- Ativan (lorazepam) Injection is available in the following dosage strengths in single-dose and multiple-dose vials:
- 2 mg per mL,
- NDC 60977-112-01, 25 ten one mL vial
- NDC 60977-116-02, 10 ten 10 mL vial
- 4 mg per mL,
- NDC 60977-113-01, 25 ten 1 mL vial
- NDC 60977-113-02, x 10 10 mL vial
- For IM or Iv injection.
Storage
- Shop in a refrigerator.
- Protect from calorie-free.
- Use carton to protect contents from light.
- Baxter is a trademark of Baxter International Inc.
- Ativan is a trademark of Biovail Laboratories International SRL.
- Manufactured by
- Baxter Healthcare Corporation
- Deerfield, IL 60015 USA
- For Production Inquiry one 800 ANA DRUG (1-800-262-3784)
- MLT01086,B
Images
Drug Images
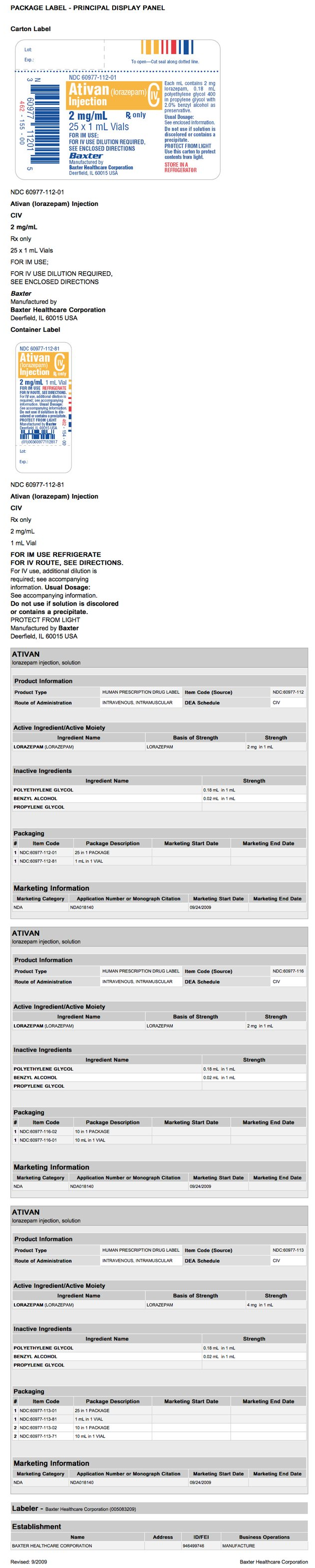
Parcel and Label Brandish Console
| |
| This image of the FDA characterization is provided past the National Library of Medicine. |
Patient Counseling Information
In that location is limited information regarding Lorazepam (injection) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Lorazepam interaction has not been established. Talk to your doctor nigh the furnishings of taking alcohol with this medication.
Brand Names
There is limited information regarding Lorazepam (injection) Brand Names in the drug label.
Look-Akin Drug Names
There is limited information regarding Lorazepam (injection) Expect-Alike Drug Names in the drug label.
Drug Shortage Status
Cost
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Greenblatt DJ, Shader RI, Franke Grand, Maclaughlin DS, Harmatz JS, Allen Physician, Werner A, Woo E (1991). "Pharmacokinetics and bioavailability of intravenous, intramuscular, and oral lorazepam in humans". Periodical of Pharmaceutical Sciences. 68 (i): 57–63. doi:x.1002/jps.2600680119. PMID 31453.
- ↑ Greenblatt DJ, von Moltke LL, Ehrenberg BL, Harmatz JS, Corbett KE, Wallace DW, Shader RI (2000). "Kinetics and dynamics of lorazepam during and later continuous intravenous infusion". Critical Intendance Medicine. 28 (8): 2750–2757. doi:10.1097/00003246-200008000-00011. PMID 10966246.
- ↑ Papini O, da Cunha SP, da Silva Mathes Ado C, Bertucci C, Moisés EC, de Barros Duarte 50, de Carvalho Cavalli R, Lanchote VL (2006). "Kinetic disposition of lorazepam with a focus on the glucuronidation capacity, transplacental transfer in parturients and racemization in biological samples". Journal of Pharmaceutical and Biomedical Assay. forty (ii): 397–403. doi:ten.1016/j.jpba.2005.07.021. PMID 16143486.
Synonyms / Brand Names: Almazine, Alzapam, Anxiedin, Aplacassee, Ativan, Bonatranquan, Delormetazepam, Emotival, Idalprem, Lorabenz, Lorax, Loraz, Lorazepam Intensol, Lorsilan, Pro Dorm, Psicopax, Punktyl, Quait, Securit, Sedatival, Sedazin, Somagerol, Tavor, Temesta, Wypax
Source: https://www.wikidoc.org/index.php/Lorazepam_(injection)
Posted by: perezbaces1957.blogspot.com


0 Response to "How To Draw Up Lorazepam Injection"
Post a Comment